MYCOBACTERIUM IN AQUACULTURE: A PERSISTENT AND COMPLEX CHALLENGE
Mycobacterium species present a significant and growing threat to the aquaculture industry, causing chronic and often fatal infections in a wide range of aquatic organisms, including fish, shrimp, and crustaceans. These hardy, slow-growing bacteria are ubiquitous in freshwater, brackish, and marine ecosystems, posing continuous challenges for aquaculture operations across various species.
The problematic nature of Mycobacterium in aquaculture stems from several factors. Firstly, these bacteria are known for their persistence in the environment and their ability to form biofilms, making them extremely difficult to eradicate once established in an aquaculture system. Their resistance to common disinfectants further complicates control efforts, often requiring extensive and costly decontamination procedures.
Mycobacterial infections are particularly insidious due to their chronic nature. The slow progression of the disease can lead to delayed detection, allowing infections to spread widely before symptoms become apparent. This characteristic not only complicates diagnosis but also makes timely intervention challenging, often resulting in significant losses before effective measures can be implemented.
The economic impact of Mycobacterium infections is substantial and multifaceted. Beyond direct mortality, infected animals often experience reduced growth rates, decreased feed conversion efficiency, and lower market value due to visible lesions or poor condition. The long-term nature of these infections can lead to sustained economic losses over extended periods, threatening the viability of aquaculture enterprises.
Another critical issue is the zoonotic potential of some Mycobacterium species, particularly M. marinum. This raises concerns about occupational health risks for aquaculture workers and potential food safety issues, which could impact consumer confidence and market access for aquaculture products.
The complex nature of mycobacterial infections presents significant challenges for treatment and control. Traditional antibiotic therapies are often ineffective due to the bacteria’s unique cell wall structure and intracellular nature. Furthermore, the prolonged treatment periods required can lead to the development of antibiotic resistance, further complicating management efforts.
As global demand for aquaculture products continues to rise, effectively addressing the Mycobacterium challenge becomes increasingly crucial for ensuring a stable and safe aquatic food supply. The industry faces the ongoing task of developing comprehensive management strategies that encompass improved biosecurity measures, enhanced detection methods, and novel treatment approaches to mitigate the impact of these persistent pathogens across all affected aquaculture species.
Common symptoms of Mycobacterium infections in fish, shrimp, and crustaceans include:
- Chronic wasting and emaciation
- Abnormal swimming behavior or lethargy
- Spinal deformities or curvature
- Skin discoloration or pale patches
- Visible granulomas or nodules on internal organs
- Exophthalmia (bulging eyes) in fish
- Scale loss or skin ulceration
- Reduced growth rates
- Decreased fertility in broodstock
- Gradual increase in mortality rates over time
These symptoms often develop slowly and can be subtle in the early stages, making early detection challenging. Regular monitoring and vigilance are crucial for effective management of mycobacterial infections in aquaculture settings.

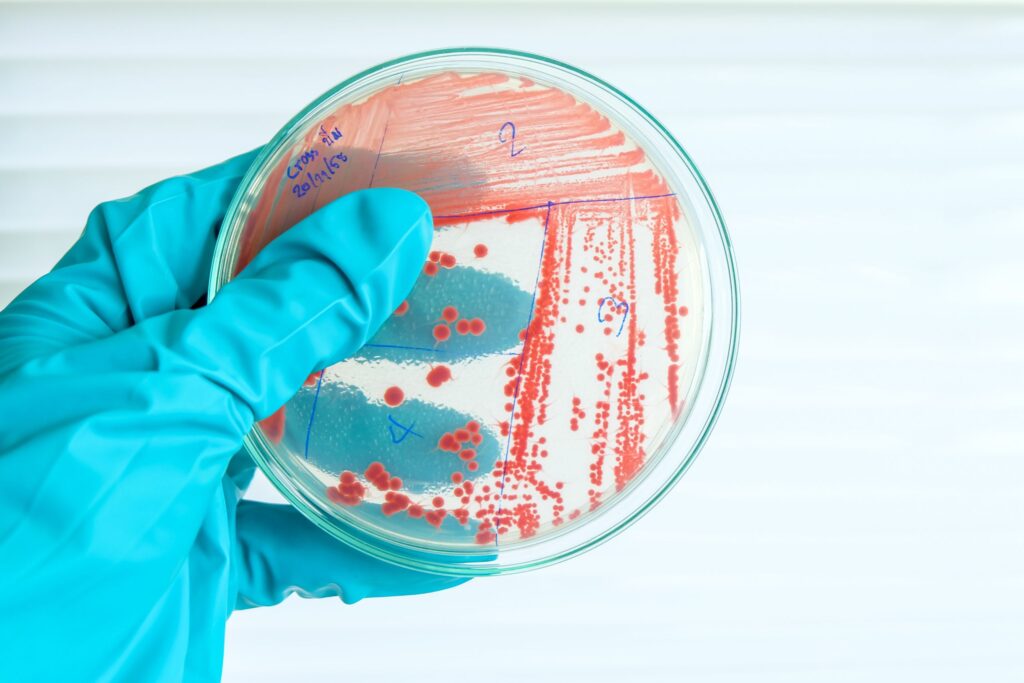
Current Limitations in Treating Mycobacterium Infections in Aquaculture
Managing Mycobacterium infections in aquaculture presents distinct challenges due to the unique characteristics of these pathogens. The slow-growing nature of mycobacteria makes early detection and timely intervention exceptionally difficult. By the time clinical signs are apparent, the infection has often spread widely within the population and contaminated the environment, complicating control efforts.
Traditional antibiotic treatments show limited efficacy against mycobacterial infections due to the bacteria’s intracellular nature and waxy cell wall. Prolonged antibiotic courses, when marginally effective, raise concerns about residues in aquaculture products and the potential development of antimicrobial resistance. Moreover, the chronic nature of these infections often requires extended treatment periods, which are impractical in most aquaculture settings.
Disinfection of mycobacteria-contaminated environments is particularly challenging. The organisms’ resilience to common disinfectants necessitates the use of harsh chemicals, which can be detrimental to the aquatic ecosystem and raise food safety concerns. This resilience also allows mycobacteria to persist in biofilms and sediments, creating reservoirs for reinfection.
Vaccine development against Mycobacterium in aquaculture faces significant obstacles. The diversity of pathogenic species affecting different aquatic organisms, combined with the bacteria’s ability to evade immune responses, complicates the creation of broadly effective vaccines. Additionally, the slow progression of infections makes it difficult to assess vaccine efficacy in field trials.
The chronic and often subclinical nature of mycobacterial infections frequently necessitates the culling of entire populations, resulting in substantial economic losses. However, this drastic measure does not address environmental contamination, leading to recurring outbreaks in restocked populations.
Water and substrate management, while crucial, are often insufficient to eliminate mycobacteria completely. In open water systems or large aquaculture operations, thorough disinfection is logistically challenging and economically burdensome. The bacteria’s ability to survive in a wide range of environmental conditions further complicates eradication efforts.
As the aquaculture industry expands, these limitations in Mycobacterium management pose significant challenges to productivity and sustainability. The need for innovative, environmentally friendly solutions that can effectively control these persistent pathogens while ensuring food safety becomes increasingly urgent.
Combat Mycobacterium with Qeen Biotechnologies' Advanced Phage Solutions. Contact Our Specialists Today for a Tailored Pathogen Management Strategy.

Bacteriophage Therapy: A Targeted Approach for Mycobacterium Control
Bacteriophage therapy presents a promising solution to the persistent challenge of Mycobacterium infections in aquaculture. This innovative approach harnesses nature’s own mechanisms to combat these notoriously difficult-to-treat pathogens, offering a targeted alternative to conventional methods that often fall short.
Unlike broad-spectrum antibiotics, bacteriophage therapy for Mycobacterium control utilizes highly specific viruses that can penetrate the bacteria’s waxy cell wall, a feature that has long hindered traditional treatments. This specificity allows for the elimination of pathogenic Mycobacterium species without disrupting the beneficial microbial communities essential for healthy aquaculture environments.
Advanced bacteriophage solutions for Mycobacterium management involve carefully selected phage cocktails, engineered to address the slow-growing nature and diverse strains of mycobacteria found in aquaculture settings. These tailored therapies are designed to maintain efficacy over the extended periods often required to combat chronic Mycobacterium infections in fish, shrimp, and other aquatic species.
The following sections will explore how bacteriophage therapy specifically tackles the unique challenges posed by Mycobacterium in aquaculture, including its ability to persist in biofilms and resist environmental stressors. This approach represents a significant advancement in aquaculture disease management, offering a potential breakthrough in controlling one of the industry’s most persistent and economically impactful pathogens.
How Bacteriophage Therapy Works Against Mycobacterium
Bacteriophage therapy functions through a precise mechanism to combat Mycobacterium in aquaculture environments. This process occurs in three key stages, targeting and eliminating harmful bacteria while preserving beneficial organisms and cultivated aquatic species.
Infection
Replication
Lysis
This process, while following the standard steps, is uniquely adapted to Mycobacterium’s characteristics, providing targeted, long-term control in aquaculture settings.
Qeen Biotechnologies
Benefits of Bacteriophage Therapy in Aquaculture
Bacteriophage therapy offers significant advantages for managing Mycobacterium infections in aquaculture, providing innovative solutions to longstanding challenges:
Unlike broad-spectrum antibiotics, bacteriophages are highly specific to their bacterial targets. This precision targeting means:
- Selective elimination of harmful Pseudomonas bacteria, preserving beneficial microbes
- Minimized disruption to the body’s natural microbiome
- Reduced risk of opportunistic infections, such as Candida albicans overgrowth
Bacteriophages provide precision in bacterial control:
- Specifically targets pathogenic Mycobacterium species, preserving beneficial microbiota
- Effective against slow-growing and intracellular Mycobacterium strains
- Minimizes disruption to the aquatic ecosystem’s microbial balance
This approach addresses the critical issue of antimicrobial resistance:
- Combats Mycobacterium strains resistant to conventional antibiotics
- Reduces reliance on long-term antibiotic use in Mycobacterium management
- Helps preserve antibiotic efficacy for both aquatic and human health applications
Bacteriophages are uniquely suited to function in Mycobacterium-prone settings:
- Functions effectively in freshwater, marine, and brackish water systems
- Maintains efficacy across various temperatures and pH levels where Mycobacterium thrive
- Adaptable to long-term treatment protocols without developing resistance
This therapy adapts to the diverse Mycobacterium challenges in aquaculture:
- Effective for various species susceptible to mycobacterial infections, including fish and crustaceans
- Can be tailored for species-specific Mycobacterium strains, such as M. marinum or M. fortuitum
- Applicable throughout different life stages vulnerable to Mycobacterium colonization
Phages excel at penetrating and disrupting Mycobacterium biofilms:
- Targets Mycobacterium within biofilms on surfaces and in sediments
- Reduces the need for harsh chemical treatments to control biofilm-associated infections
- Helps maintain water quality by managing Mycobacterium populations in system components
This method supports sustainable Mycobacterium control practices:
- Minimal impact on non-target organisms and surrounding ecosystems
- Aids in breaking the cycle of environmental contamination and reinfection
- Reduces the risk of zoonotic transmission to aquaculture workers
Bacteriophage therapy contributes to improved aquaculture productivity:
- Potential for improved growth rates by managing chronic Mycobacterium infections
- Reduces long-term mortality rates associated with persistent Mycobacterium presence
- Lowers production costs by decreasing reliance on expensive treatments and facility decontamination
These benefits highlight bacteriophage therapy’s potential as an innovative approach to Mycobacterium control in aquaculture. By providing a targeted, environmentally responsible, and efficient solution, this advanced treatment addresses critical Mycobacterium-related challenges while promoting sustainable practices and improved productivity in the face of this persistent pathogen.
Regulatory Expertise for Mycobacterium Phage Therapy
Qeen Biotechnologies provides regulatory support for bacteriophage therapy targeting Mycobacterium in aquaculture. Our team navigates the regulatory landscape surrounding phage-based treatments for these aquatic pathogens. We assist throughout the approval process, from initial consultations on Mycobacterium-specific phage applications to the preparation of regulatory documentation.
Our expertise in Chemistry, Manufacturing, and Controls (CMC) supports the development of compliant manufacturing processes for Mycobacterium-targeting bacteriophages. We address the challenges of introducing novel biologics for chronic infections in aquaculture systems, aligning strategies with global regulatory frameworks while considering the requirements of long-term Mycobacterium control.
We work with clients to develop approaches that meet the requirements of various regulatory bodies. This helps optimize the approval process for Mycobacterium phage therapies. We provide guidance on safety and efficacy studies specific to Mycobacterium phage therapy in aquaculture, including long-term study designs, data collection methodologies, and result interpretation for regulatory submissions.
Our regulatory expertise enables aquaculture professionals to implement bacteriophage solutions for Mycobacterium control that comply with regulatory standards. This support is particularly relevant in addressing the challenges posed by Mycobacterium infections in aquaculture, aiding the industry’s adoption of new treatment options.
Tailored Bacteriophage Solutions for Mycobacterium Control
Qeen Biotechnologies develops customized bacteriophage solutions to address Mycobacterium infections in aquaculture. Our process begins with analysis of client-specific Mycobacterium strains, using genomic sequencing and phenotypic characterization. This profiling enables us to identify effective phages for targeting the particular Mycobacterium variants in each aquaculture system.
We create phage cocktails for different aquatic species, considering the varied manifestations of Mycobacterium infections across fish, crustaceans, and mollusks. Our approach takes into account factors such as the host species’ physiology, immune response, and environmental conditions to optimize phage efficacy and safety against Mycobacterium.
Our team develops solutions for specific Mycobacterium-related challenges, including chronic infections, biofilm-associated colonization, and antibiotic-resistant strains. The process involves ongoing monitoring and refinement of phage formulations to maintain efficacy against evolving Mycobacterium populations.
We employ bioengineering techniques to enhance phage stability and delivery in aquaculture environments where Mycobacterium persists. Our formulation expertise ensures that treatments remain effective under diverse water conditions, temperatures, and application methods specific to each client’s Mycobacterium control needs.
By integrating phage technology with aquaculture industry knowledge, Qeen Biotechnologies provides engineered solutions for Mycobacterium control. This approach aims to enhance treatment efficacy while supporting sustainable aquaculture practices in managing Mycobacterium-related challenges.
Connect with Our Experts
Experiencing challenges with bacterial infections? Our team at Qeen Biotechnologies specializes in developing and producing bacteriophage-based therapies across various industries. Contact us to learn how our innovative approaches can address your specific needs.





